Defining the digital patient experience at a large genomic testing company.
The day a person is diagnosed with cancer, they (and their loved ones) are thrown into a world of terminology, procedures, emotional strain, and financial burdens. As a provider of genomic profiling tests, Foundation Medicine understands it is a only a moment in a patient's whole journey, but it is also potentially a life-saving one. Because tests are ordered by oncologists, however, many patients are overwhelmed by their diagnosis, and remain oblivious to what genomic profiling is and how it impacts them.
While we don't want to interfere in the provider-patient relationship, new evidence has shown that patients are becoming increasingly educated about their disease, attentive to their own data, and involved in decision-making about their own care. Those that are, can even see better clinical outcomes as a result¹.
¹ JAMA Oncol. 2019;5(12):1689-1690. doi:10.1001/jamaoncol.2019.4390
The Challenge
Foundation Medicine had no strategy for direct-to-patient engagement, save a few scattered touch-points that lacked a shared strategic focus. There were no digital products or services built specifically for patients to explore their own genomic data, or engage on a deeper level with Foundation Medicine's knowledge-base. In a worst-case scenario, a patient's first introduction to the company was sometimes a bill.
Our objective was to propose a digital patient experience that would align the company's patient touch-points around a unified strategy, and lay a foundation for deeper direct-to-patient engagement.
My Role
I designed a multi phase research study, managing one senior designer to support the creation of testing concepts. As leader, I hired vendors to recruit patients, ran generative research sessions with patients, synthesized feedback, and organized regular sharebacks with stakeholders.
In a later phase, I managed the design of a new digital patient experience, which led to further rounds of formative testing. The final deliverables included patient archetypes, journey maps, and a proposal to our company's portfolio committee.
Business Goals
Evaluate the feasibility of DTP applications that could improve experience and outcomes.
Align internal efforts around patient engagement.
Evaluate key internal hypotheses around product-based engagement
Document and socialize drivers of patient experience, barriers to care access, key challenges and needs.
Discovery
01 – Generative Research
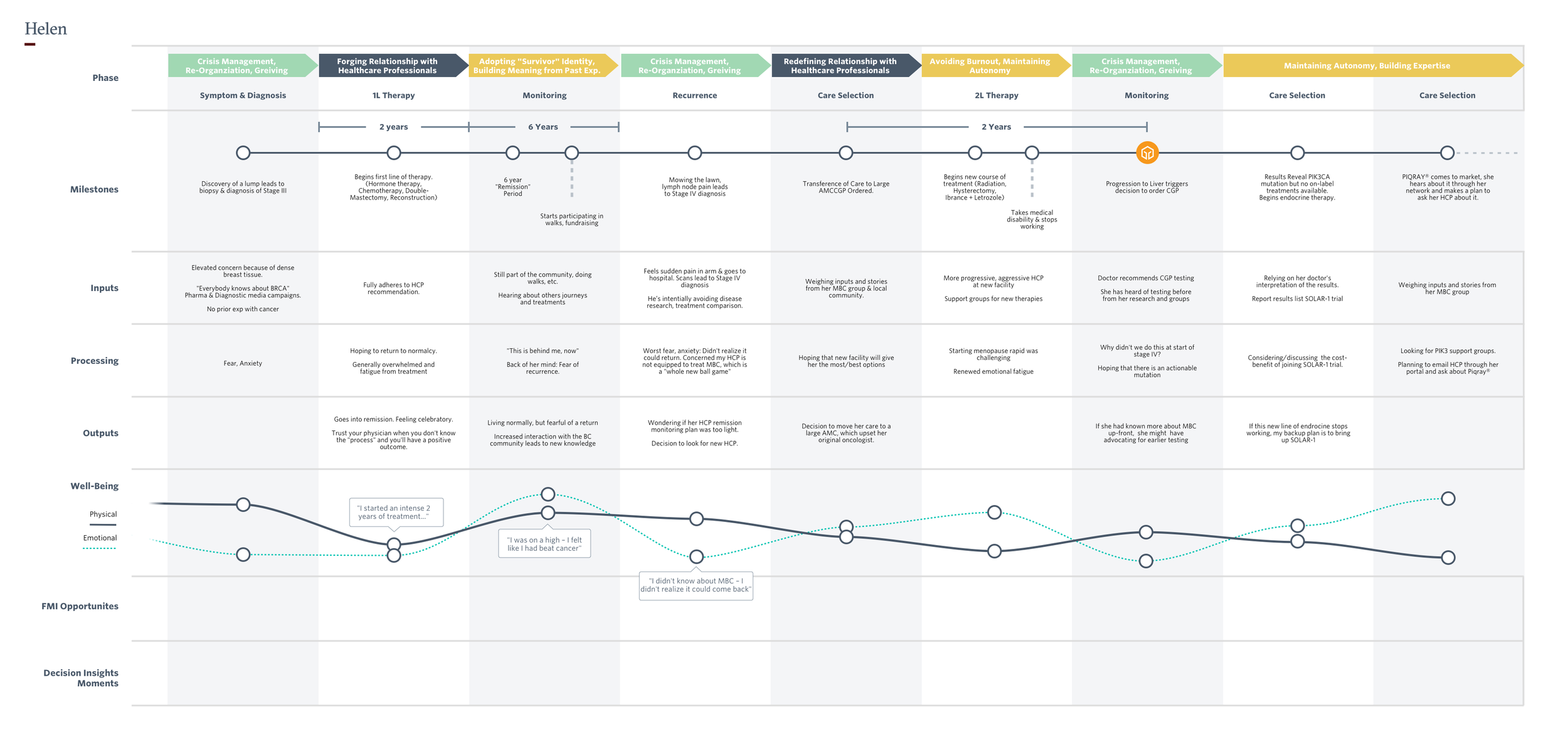
I screened, recruited, and ran one-hour remote interviews with around 20 advanced cancer patients (active and in remission), across Foundation Medicines key tumor types. I heard and documented, among many things, cancer stories, experiences with genomic testing, relationships to providers, systems of support, and attitudes toward clinical research.
-
Balancing recruitment bias – research vendors overindexed on white, educated women. Breast and Lung cancer patients tend to be the most involved in community research. Special consideration was paid to recruiting men and people of color.
-
Four patient archetypes, comprised of the most commonly repeating personal and environmental factors.
Sample Journey maps that detail emotional, educational and clinical milestones.
Opportunity areas for patient engagement
02 – Due Diligence
I ran secondary research with internal teams that managed patient touchpoints including our billing, client services, and patient advocacy teams. My goal was gather their insight, but also locate any upcoming or unknown direct-to-patient initiatives. With my product manager counterpart, I ran deep dives on potential opportunity areas with the commercial team, clinical operations, and payer strategy teams. I also led workshops with our "Patient Community Council", a group of highly engaged patient advocates and thought-leaders.
-
Discovery of upcoming patient experience efforts happening in silos.
Aligning several teams goals and schedules.
-
A list of internal teams and points of contact to keep updated on our efforts.
A “steering committee” for patient work across the company.
Onsite work to build/plan archetypes.
A collection of patient archetypes
4x4 plot, detailing the axes used to define archetypes and demonstrating how they can change over time.
In-progress map of a generalized patient “treatment journey”.
Delivery
Patient & Provider Testing
When enough data was gathered and synthesized, my team created testing stimuli, designed to collect feedback on several key product hypotheses. The stimuli focused around several key areas: "First Touch" (physical and digital first encounters with the company), entry points into a digital product, experience while awaiting results delivery, the ultimate nature of results delivery (direct to patient vs. through provider), relationship to Foundation after testing, and willingness to donate de-indentified data for clinical research.
I also interviewed 10 oncologists and showed them the same material for validation & acceptance testing.
-
Managing emotionally charged conversations and creating space for meaningful stories, while keeping interviews on track and achieving our goals.
Balancing patient desires with provider (customer) apprehension. For example: Whether to release data directly to patients, or first to providers.
-
Lists of features that are high-priority to patient care, organized by value to the business.
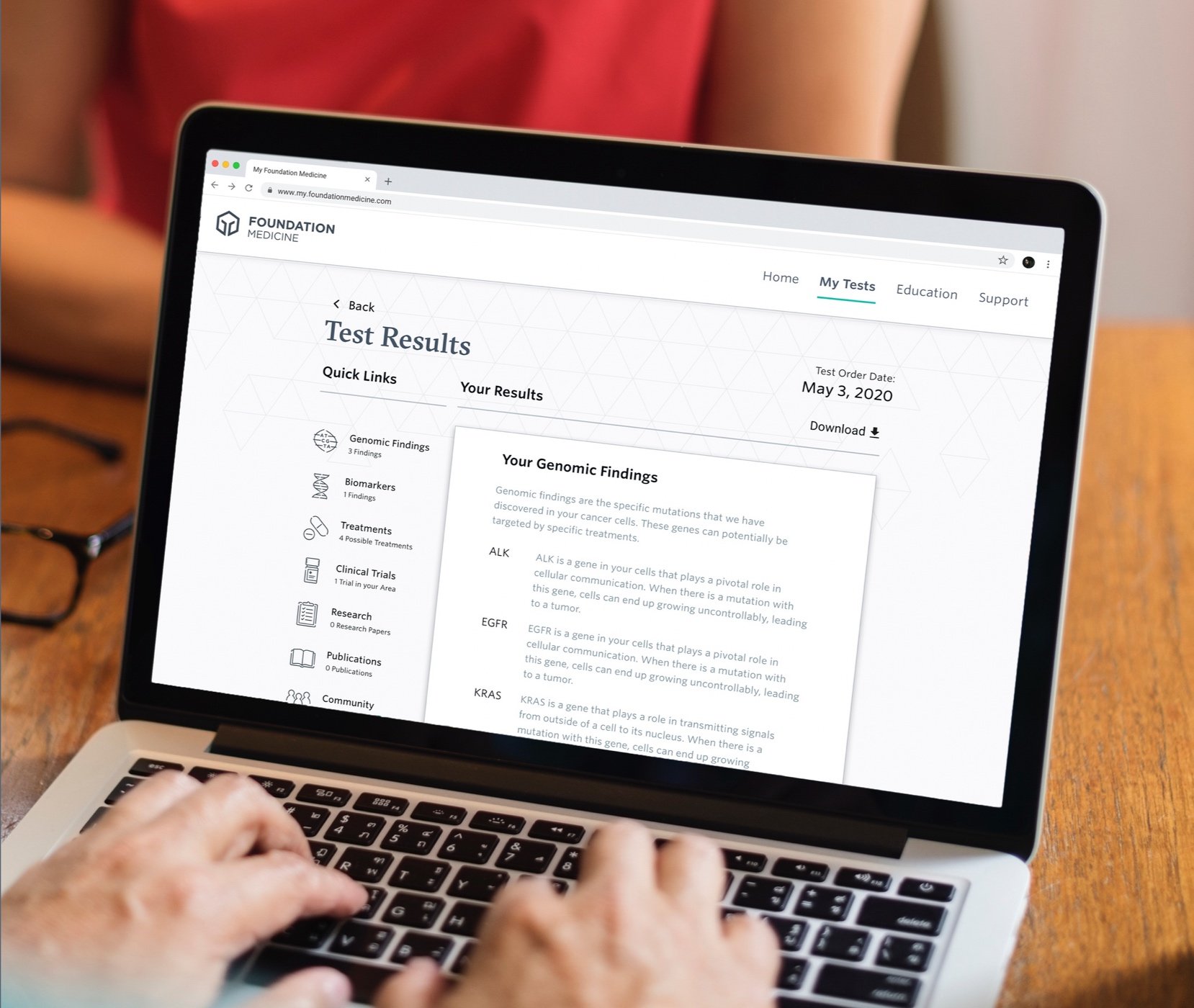
A clickable prototype for a patient portal, reflective of research findings.
Testing Stimuli: Results Delivery
Testing Stimuli: Test Tracker
Testing Stimuli: Response to Processing Issues
Additional Activities
After building a reputation as “the patient person”, I was contacted by additional teams to bring my insight about the patient experience to a number of projects and activities.
I conducted several interactive workshops with our Patient Community Council to dive deep on a number of topics, including minority representation in clinical trials, patient-friendly reports, and barriers to test access.
I supported a collaboration between our Clinical Operations team and a patient advocacy org, to author survey materials designed to collect data on patient experience during a clinical trial.